Page 137 - Science Course 2 (Book 1)
P. 137
Covalent Bond Mo5-L2a: What are Covalent Compounds?
Lesson 2
Let’s Begin
In this topic, you will learn the following
lessons: From Elements to Compounds
Mo.5–L2a: What are Covalent Compounds? Compounds are chemical combinations of different
Mo.5–L2b: What is a Polar Molecule? types of atoms.
Chemical bonds join atoms together.
Mo5-L2a What are Covalent Compounds?
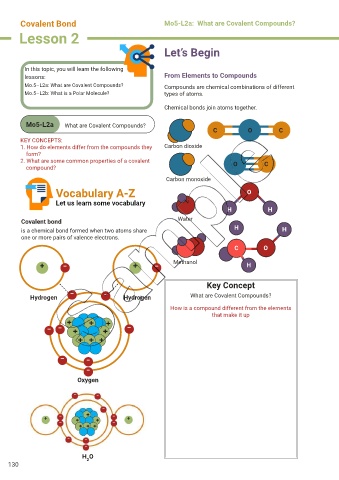
C O C
KEY CONCEPTS:
1. How do elements differ from the compounds they Carbon dioxide
form?
2. What are some common properties of a covalent O C
compound?
Carbon monoxide
Vocabulary A-Z O
Let us learn some vocabulary
H H
Covalent bond Water
is a chemical bond formed when two atoms share H H
one or more pairs of valence electrons.
C O
+ – + – Methanol H
Key Concept
– –
Hydrogen Hydrogen What are Covalent Compounds?
How is a compound different from the elements
that make it up
– – + + + + + –
+
+
+
– –
–
Oxygen
H O
2
130