Page 16 - Science Course 1 (Book 2)
P. 16
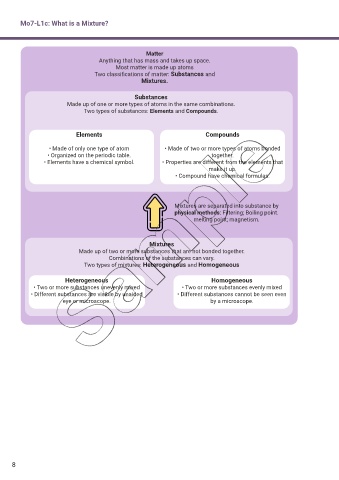
Mo7-L1c: What is a Mixture?
Matter
Anything that has mass and takes up space.
Most matter is made up atoms
Two classif cations of matter: Substances and
Mixtures.
Substances
Made up of one or more types of atoms in the same combinations.
Two types of substances: Elements and Compounds.
Elements Compounds
• Made of only one type of atom • Made of two or more types of atoms bonded
• Organized on the periodic table. together.
• Elements have a chemical symbol. • Properties are different from the elements that
make it up.
• Compound have chemical formulas.
Mixtures are separated into substance by
physical methods: Filtering; Boiling point.
melting point; magnetism.
Mixtures
Made up of two or more substances that are not bonded together.
Combinations of the substances can vary.
Two types of mixtures: Heterogeneous and Homogeneous
Heterogeneous Homogeneous
• Two or more substances unevenly mixed • Two or more substances evenly mixed
• Different substances are visible by unaided • Different substances cannot be seen even
eye or microscope. by a microscope.
8