Page 64 - Science Course 2 (Book 2)
P. 64
Mo9-L3b: What are the Different Forms of Energy?
Let’s Begin When fossil fuels burn, the chemical bonds between
the atoms that make up the fossil fuel break apart.
When this happens, chemical energy transforms to
Types of Energy thermal energy.
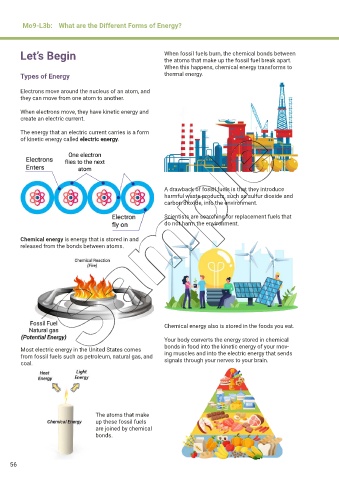
Electrons move around the nucleus of an atom, and
they can move from one atom to another.
When electrons move, they have kinetic energy and
create an electric current.
The energy that an electric current carries is a form
of kinetic energy called electric energy.
A drawback of fossil fuels is that they introduce
harmful waste products, such as sulfur dioxide and
carbon dioxide, into the environment.
Scientists are searching for replacement fuels that
do not harm the environment.
Chemical energy is energy that is stored in and
released from the bonds between atoms.
Chemical energy also is stored in the foods you eat.
Your body converts the energy stored in chemical
bonds in food into the kinetic energy of your mov-
Most electric energy in the United States comes ing muscles and into the electric energy that sends
from fossil fuels such as petroleum, natural gas, and signals through your nerves to your brain.
coal.
The atoms that make
up these fossil fuels
are joined by chemical
bonds.
56