Page 24 - Science Course 1 (Book 2)
P. 24
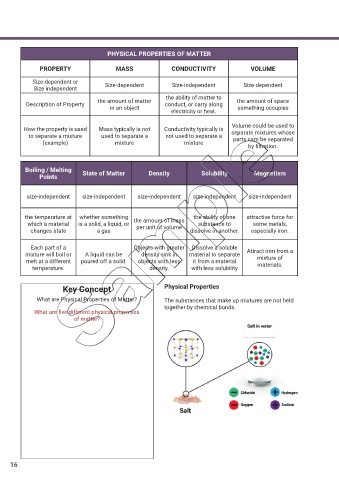
PHYSICAL PROPERTIES OF MATTER
PROPERTY MASS CONDUCTIVITY VOLUME
Size-dependent or Size-dependent Size-independent Size-dependent
Size independent
the ability of matter to
the amount of matter the amount of space
Description of Property conduct, or carry along
in an object something occupies
electricity or heat.
Volume could be used to
How the property is used Mass typically is not Conductivity typically is separate mixtures whose
to separate a mixture used to separate a not used to separate a parts cam be separated
(example) mixture mixture
by f ltration.
Boiling / Melting State of Matter Density Solubility Magnetism
Points
size-independent size-independent size-independent size-independent size-independent
the temperature at whether something the amount of mass the ability of one attractive force for
which a material is a solid, a liquid, or per unit of volume substance to some metals,
changes state a gas dissolve in another. especially iron.
Each part of a Objects with greater Dissolve a soluble
mixture will boil or A liquid can be density sink in material to separate Attract iron from a
mixture of
melt at a different poured off a solid. objects with less it from a material materials.
temperature. density. with less solubility
Key Concept Physical Properties
What are Physical Properties of Matter? The substances that make up mixtures are not held
together by chemical bonds.
What are f ve different physical properties
of matter?
16