Page 102 - Science Course 2 (Book 1)
P. 102
Mo4-L1a: What are the Properties of a Solution?
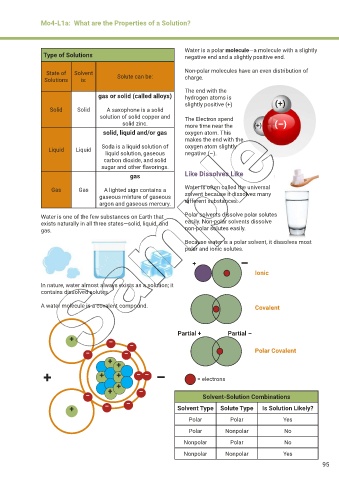
Water is a polar molecule—a molecule with a slightly
Type of Solutions negative end and a slightly positive end.
State of Solvent Non-polar molecules have an even distribution of
Solutions is: Solute can be: charge.
The end with the
gas or solid (called alloys) hydrogen atoms is
slightly positive (+) (+)
Solid Solid A saxophone is a solid
solution of solid copper and The Electron spend
solid zinc. more time near the (+) (–)
solid, liquid and/or gas oxygen atom. This
makes the end with the
Liquid Liquid Soda is a liquid solution of oxygen atom slightly
liquid solution, gaseous negative (–).
carbon dioxide, and solid
sugar and other flavorings.
gas Like Dissolves Like
Gas Gas A lighted sign contains a Water is often called the universal
gaseous mixture of gaseous solvent because it dissolves many
argon and gaseous mercury. different substances.
Water is one of the few substances on Earth that Polar solvents dissolve polar solutes
exists naturally in all three states—solid, liquid, and easily. Non-polar solvents dissolve
gas. non-polar solutes easily.
Because water is a polar solvent, it dissolves most
polar and ionic solutes.
+ –
Ionic
In nature, water almost always exists as a solution; it
contains dissolved solutes.
A water molecule is a covalent compound. Covalent
Partial + Partial –
+ –
–
– – Polar Covalent
+ +
+ + + – – – = electrons
+ + –
– Solvent-Solution Combinations
+ – – Solvent Type Solute Type Is Solution Likely?
Polar Polar Yes
Polar Nonpolar No
Nonpolar Polar No
Nonpolar Nonpolar Yes
95