Page 104 - Science Course 2 (Book 1)
P. 104
Mo4-L1b: What are Solubility and Concentration?
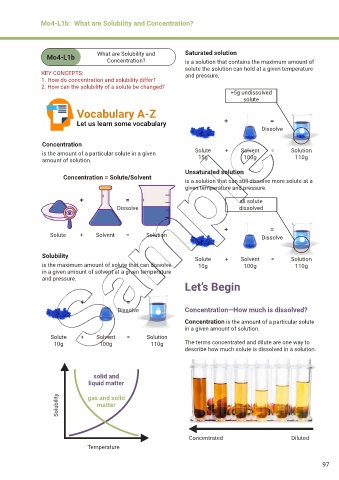
Mo4-L1b What are Solubility and Saturated solution
Concentration?
is a solution that contains the maximum amount of
solute the solution can hold at a given temperature
KEY CONCEPTS: and pressure.
1. How do concentration and solubility differ?
2. How can the solubility of a solute be changed?
+5g undissolved
solute
Vocabulary A-Z
Let us learn some vocabulary + =
Dissolve
Concentration
is the amount of a particular solute in a given Solute + Solvent = Solution
110g
100g
15g
amount of solution.
Unsaturated solution
Concentration = Solute/Solvent
is a solution that can still dissolve more solute at a
given temperature and pressure.
+ = all solute
Dissolve dissolved
+ =
Solute + Solvent = Solution Dissolve
Solubility Solute + Solvent = Solution
is the maximum amount of solute that can dissolve 10g 100g 110g
in a given amount of solvent at a given temperature
and pressure.
Let’s Begin
+ =
Dissolve Concentration—How much is dissolved?
Concentration is the amount of a particular solute
in a given amount of solution.
Solute + Solvent = Solution
10g 100g 110g The terms concentrated and dilute are one way to
describe how much solute is dissolved in a solution.
solid and
liquid matter
Solubility gas and solid
matter
Concentrated Diluted
Temperature
97