Page 130 - Science Course 2 (Book 1)
P. 130
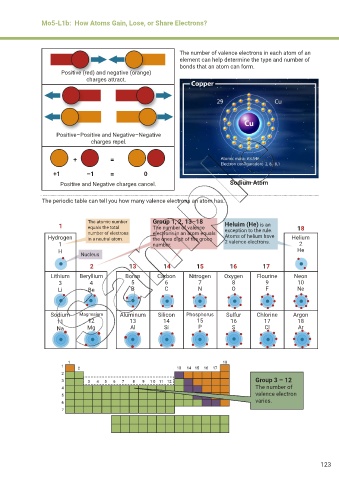
Mo5-L1b: How Atoms Gain, Lose, or Share Electrons?
The number of valence electrons in each atom of an
element can help determine the type and number of
bonds that an atom can form.
Positive (red) and negative (orange)
charges attract.
Positive–Positive and Negative–Negative
charges repel.
+ =
+1 –1 = 0
Positive and Negative charges cancel. Sodium Atom
The periodic table can tell you how many valence electrons an atom has.
The atomic number Group 1, 2, 13–18
1 equals the total The number of valence Heluim (He) is an 18
number of electrons electrons in an atom equals exception to the rule.
Hydrogen in a neutral atom. the ones digit of the group Atoms of helium have Helium
1 number. 2 valence electrons. 2
H He
Nucleus
2 13 14 15 16 17
Lithium Beryllium Boron Carbon Nitrogen Oxygen Flourine Neon
3 4 5 6 7 8 9 10
Li Be B C N O F Ne
Sodium Magnesium Aluminum Silicon Phosphorus Sulfur Chlorine Argon
11 12 13 14 15 16 17 18
Na Mg Al Si P S Cl Ar
Group 3 – 12
The number of
valence electron
varies.
123