Page 131 - Science Course 2 (Book 1)
P. 131
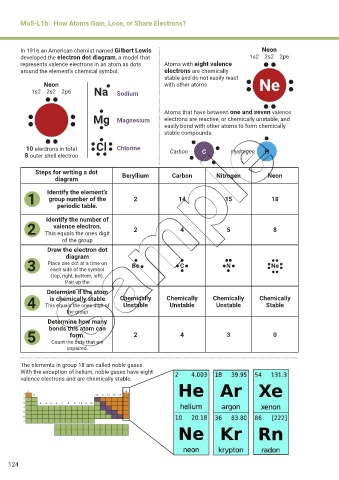
Mo5-L1b: How Atoms Gain, Lose, or Share Electrons?
In 1916 an American chemist named Gilbert Lewis Neon
developed the electron dot diagram, a model that 1s2 2s2 2p6
represents valence electrons in an atom as dots Atoms with eight valence
around the element’s chemical symbol. electrons are chemically
stable and do not easily react
Neon with other atoms Ne
1s2 2s2 2p6 Na Sodium
Atoms that have between one and seven valence
Mg Magnesium electrons are reactive, or chemically unstable, and
easily bond with other atoms to form chemically
stable compounds.
Cl
10 electrons in total Chlorine Carbon C Hydrogen H
8 outer shell electron
Steps for writing a dot Beryllium Carbon Nitrogen Neon
diagram
1 Identify the element’s 2 14 15 18
group number of the
periodic table.
Identify the number of
2 This equals the ones digit 2 4 5 8
valence electron.
of the group
Draw the electron dot
diagram
3 Place one dot at a time on Be C N Ne
each side of the symbol
(top, right, bottom, left).
Pair up the
Determine if the atom
4 This equals the ones digit of Chemically Chemically Chemically Chemically
is chemically stable
Unstable
Unstable
Stable
Unstable
the group
Determine how many
5 bonds this atom can 2 4 3 0
form.
Count the dots that are
unpaired.
The elements in group 18 are called noble gases.
With the exception of helium, noble gases have eight
valence electrons and are chemically stable.
124