Page 273 - Science Course 3 (Book 1)
P. 273
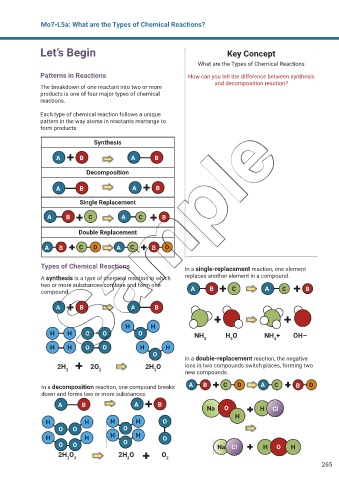
Mo7-L5a: What are the Types of Chemical Reactions?
Let’s Begin Key Concept
What are the Types of Chemical Reactions
Patterns in Reactions How can you tell the difference between synthesis
and decomposition reaction?
The breakdown of one reactant into two or more
products is one of four major types of chemical
reactions.
Each type of chemical reaction follows a unique
pattern in the way atoms in reactants rearrange to
form products.
Synthesis
A B A B
Decomposition
A B A B
Single Replacement
A B C A C B
Double Replacement
A B C D A C B D
Types of Chemical Reactions In a single-replacement reaction, one element
A synthesis is a type of chemical reaction in which replaces another element in a compound.
two or more substances combine and form one
compound. A B C A C B
A B A B
H H
H H O O O NH H O NH + OH–
3 3 4
H H O O H H
O
In a double-replacement reaction, the negative
2H 2O 2H O ions in two compounds switch places, forming two
2 2 2 new compounds.
In a decomposition reaction, one compound breaks A B C D A C B D
down and forms two or more substances.
A B A B Na O H Cl
H
H H H H O
O O O
H H H H O
O O O Na Cl H O H
2H O 2H O O
2 2 2 2
265