Page 272 - Science Course 3 (Book 1)
P. 272
Chemical Reactions Mo7-L5a: What are the Types of Chemical
Lesson 5 Reactions?
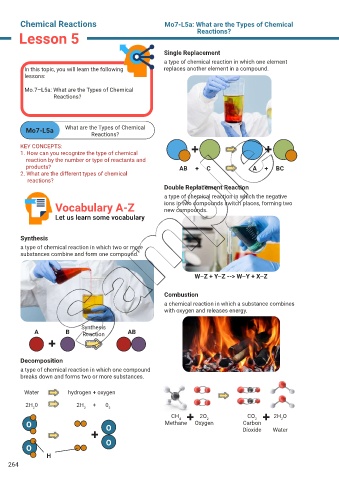
Single Replacement
a type of chemical reaction in which one element
In this topic, you will learn the following replaces another element in a compound.
lessons:
Mo.7–L5a: What are the Types of Chemical
Reactions?
Mo7-L5a What are the Types of Chemical
Reactions?
KEY CONCEPTS:
1. How can you recognize the type of chemical
reaction by the number or type of reactants and
products? AB + C A + BC
2. What are the different types of chemical
reactions?
Double Replacement Reaction
a type of chemical reaction in which the negative
Vocabulary A-Z ions in two compounds switch places, forming two
new compounds.
Let us learn some vocabulary
Synthesis
a type of chemical reaction in which two or more
substances combine and form one compound.
W–Z + Y–Z --> W–Y + X–Z
Combustion
a chemical reaction in which a substance combines
with oxygen and releases energy.
Synthesis
A B Reaction AB
Decomposition
a type of chemical reaction in which one compound
breaks down and forms two or more substances.
Water hydrogen + oxygen
2H 0 2H 2 + 0 2
2
CH 4 2O 2 CO 2 2H O
2
Carbon
O O Methane Oxygen Dioxide Water
O
O
H
264