Page 22 - Science Course 3 (Book 2)
P. 22
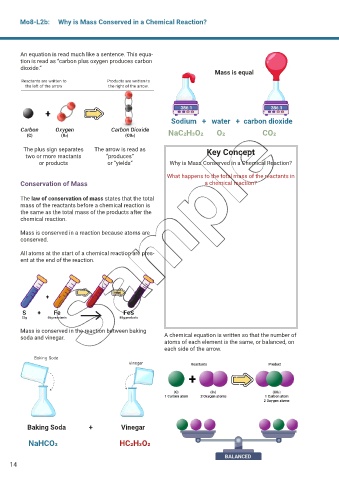
Mo8-L2b: Why is Mass Conserved in a Chemical Reaction?
An equation is read much like a sentence. This equa-
tion is read as “carbon plus oxygen produces carbon
dioxide.”
Mass is equal
Sodium + water + carbon dioxide
NaC2H3O2 O2 CO2
The plus sign separates The arrow is read as Key Concept
two or more reactants “produces”
or products or “yields” Why is Mass Conserved in a Chemical Reaction?
What happens to the total mass of the reactants in
Conservation of Mass a chemical reaction?
The law of conservation of mass states that the total
mass of the reactants before a chemical reaction is
the same as the total mass of the products after the
chemical reaction.
Mass is conserved in a reaction because atoms are
conserved.
All atoms at the start of a chemical reaction are pres-
ent at the end of the reaction.
Mass is conserved in the reaction between baking
soda and vinegar. A chemical equation is written so that the number of
atoms of each element is the same, or balanced, on
each side of the arrow.
Baking Soda + Vinegar
NaHCO3 HC2H3O2
14