Page 23 - Science Course 3 (Book 2)
P. 23
Mo8-L2b: Why is Mass Conserved in a Chemical Reaction?
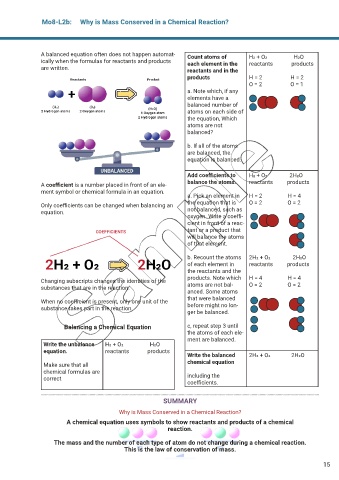
A balanced equation often does not happen automat- Count atoms of H2 + O2 ͢ H2O
ically when the formulas for reactants and products each element in the reactants products
are written. reactants and in the
products H = 2 H = 2
O = 2 O = 1
a. Note which, if any
elements have a
balanced number of
atoms on each side of
the equation, Which
atoms are not
balanced?
b. If all of the atoms
are balanced, the
equation is balanced.
Add coef cients to H2 + O2 ͢ 2H2O
A coef cient is a number placed in front of an ele- balance the atoms. reactants products
ment symbol or chemical formula in an equation.
a. Pick an element in H = 2 H = 4
Only coef cients can be changed when balancing an the equation that is O = 2 O = 2
equation. not balanced, such as
oxygen. Write a coef -
cient in front of a reac-
tant or a product that
will balance the atoms
of that element.
b. Recount the atoms 2H2 + O2 ͢ 2H2O
of each element in reactants products
the reactants and the
products. Note which H = 4 H = 4
Changing subscripts changes the identities of the
substances that are in the reaction. atoms are not bal- O = 2 O = 2
anced. Some atoms
that were balanced
When no coef cient is present, only one unit of the
substance takes part in the reaction. before might no lon-
ger be balanced.
Balancing a Chemical Equation c, repeat step 3 until
the atoms of each ele-
ment are balanced.
Write the unbalance H2 + O2 ͢ H2O
equation. reactants products
Write the balanced 2H2 + O2 ͢ 2H2O
Make sure that all chemical equation
chemical formulas are
correct including the
coef cients.
SUMMARY
Why is Mass Conserved in a Chemical Reaction?
A chemical equation uses symbols to show reactants and products of a chemical
reaction.
The mass and the number of each type of atom do not change during a chemical reaction.
This is the law of conservation of mass.
15