Page 163 - Science Course 1 (Book 1)
P. 163
Matter: Movement of Particles Mo5-L2a: How Do Particles Move in the
Different States of Matter?
Lesson 2
Let’s Begin
In this topic, you will learn the following
lessons: Describing Matter
Mo.5 - L2a: How Do Particles Move in the Two factors determine the state of matter:
Different States of Matter?
Mo.5 - L2b: How Do Particles Move in Solids, 1. The motion of the particles of the matter
Liquids, and Gases? 2. The forces between the particles of the matter
Mo5-L2a How Do Particles Move in the
Different States of Matter?
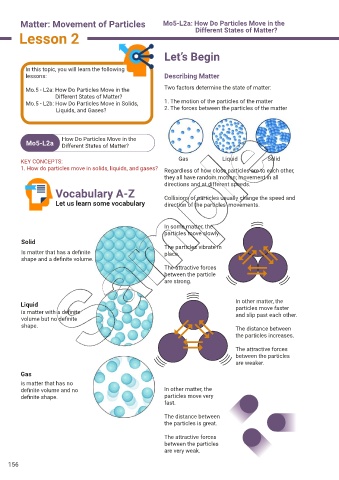
KEY CONCEPTS: Gas Liquid Solid
1. How do particles move in solids, liquids, and gases? Regardless of how close particles are to each other,
they all have random motion; movement in all
directions and at different speeds.
Vocabulary A-Z
Let us learn some vocabulary Collisions of particles usually change the speed and
direction of the particles’ movements.
In some matter, the
particles move slowly.
Solid
The particles vibrate in
Is matter that has a definite place.
shape and a definite volume.
The attractive forces
between the particle
are strong.
Liquid In other matter, the
particles move faster
is matter with a definite and slip past each other.
volume but no definite
shape. The distance between
the particles increases.
The attractive forces
between the particles
are weaker.
Gas
is matter that has no
definite volume and no In other matter, the
definite shape. particles move very
fast.
The distance between
the particles is great.
The attractive forces
between the particles
are very weak.
156