Page 166 - Science Course 1 (Book 1)
P. 166
Mo5-L2b: How Do Particles Move in Solids, Liquids, and Gases?
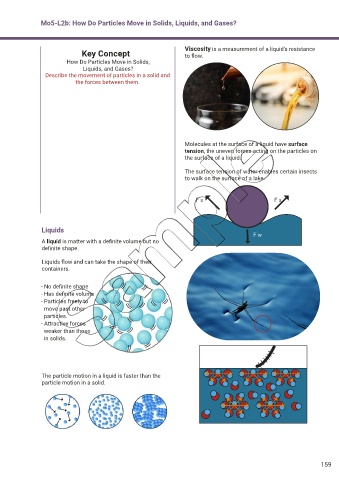
Key Concept Viscosity is a measurement of a liquid’s resistance
to flow.
How Do Particles Move in Solids,
Liquids, and Gases?
Describe the movement of particles in a solid and
the forces between them.
Molecules at the surface of a liquid have surface
tension, the uneven forces acting on the particles on
the surface of a liquid.
The surface tension of water enables certain insects
to walk on the surface of a lake.
F s F s
Liquids
F w
A liquid is matter with a definite volume but no
definite shape.
Liquids flow and can take the shape of their
containers.
- No definite shape
- Has definite volume
- Particles freely to
move past other
particles.
- Attractive forces
weaker than those
in solids.
The particle motion in a liquid is faster than the
particle motion in a solid.
159