Page 20 - Science Course 1 (Book 1)
P. 20
Mo. 1 - L2b What are precision and Accuracy?
Mo1-L2b What are precision and Accuracy?
KEY CONCEPTS:
1. How do accuracy and precision differ?
2. Why should you use significant digits? Precision is a
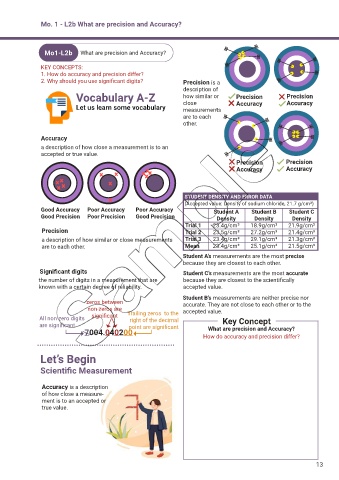
description of
Vocabulary A-Z how similar or Precision Precision
Let us learn some vocabulary close Accuracy Accuracy
measurements
are to each
other.
Accuracy
a description of how close a measurement is to an
accepted or true value.
Precision Precision
Accuracy Accuracy
STUDENT DENSITY AND ERROR DATA
(Accepted value: Density of sodium chloride, 21.7 g/cm³)
Good Accuracy Poor Accuracy Poor Accuracy Student A Student B Student C
Good Precision Poor Precision Good Precision Density Density Density
Trial 1 23.4g/cm³ 18.9g/cm³ 21.9g/cm³
Precision Trial 2 23.5g/cm³ 27.2g/cm³ 21.4g/cm³
a description of how similar or close measurements Trial 3 23.4g/cm³ 29.1g/cm³ 21.3g/cm³
are to each other. Mean 23.4g/cm³ 25.1g/cm³ 21.5g/cm³
Student A’s measurements are the most precise
because they are closest to each other.
Significant digits Student C’s measurements are the most accurate
the number of digits in a measurement that are because they are closest to the scientifically
known with a certain degree of reliability. accepted value.
Student B’s measurements are neither precise nor
zeros between accurate. They are not close to each other or to the
non-zeros are Trailing zeros to the accepted value.
All non-zero digits significant right of the decimal Key Concept
are significant point are significant What are precision and Accuracy?
7004.040200 How do accuracy and precision differ?
Let’s Begin
Scientific Measurement
Accuracy is a description
of how close a measure-
ment is to an accepted or
true value.
13