Page 224 - Science Course 1 (Book 1)
P. 224
Mo6-L4b: How are Elements Arranged on the Periodic Table?
Key Concept
How are Elements Arranged on the
Periodic Table?
What can you infer about the properties of two
elements in the same group?
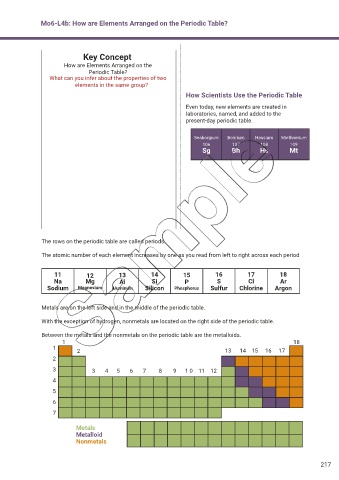
How Scientists Use the Periodic Table
Even today, new elements are created in
laboratories, named, and added to the
present-day periodic table.
Seaborgium Bohrium Hassium Miethnerium
106 107 108 109
Sg Bh Hs Mt
The rows on the periodic table are called periods.
The atomic number of each element increases by one as you read from left to right across each period
11 12 13 14 15 16 17 18
Na Mg Al Si P S Cl Ar
Sodium Magnesium Aluminum Silicon Phosphorus Sulfur Chlorine Argon
Metals are on the left side and in the middle of the periodic table.
With the exception of hydrogen, nonmetals are located on the right side of the periodic table.
Between the metals and the nonmetals on the periodic table are the metalloids.
1 18
1
2 13 14 15 16 17
2
3 3 4 5 6 7 8 9 1 0 11 12
4
5
6
7
Metals
Metalloid
Nonmetals
217