Page 221 - Science Course 1 (Book 1)
P. 221
The Periodic Table Mo6-L4a: What is The Periodic Table?
Lesson 4
In this topic, you will learn the following
lessons: Let’s Begin
Mo.6-L4a: What is The Periodic Table?
Mo.6-L4b: How are Elements Arranged on
the Periodic Table? What is the periodic table?
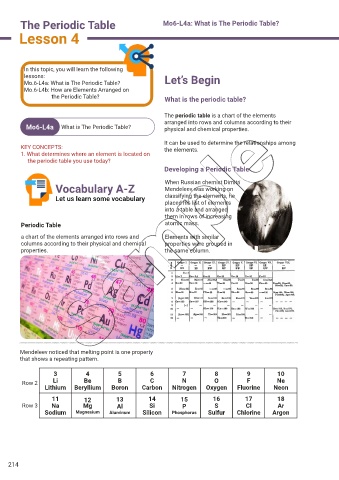
The periodic table is a chart of the elements
arranged into rows and columns according to their
Mo6-L4a What is The Periodic Table? physical and chemical properties.
It can be used to determine the relationships among
KEY CONCEPTS: the elements.
1. What determines where an element is located on
the periodic table you use today?
Developing a Periodic Table
Vocabulary A-Z When Russian chemist Dimitri
Mendeleev was working on
Let us learn some vocabulary classifying the elements, he
placed his list of elements
into a table and arranged
them in rows of increasing
Periodic Table atomic mass.
a chart of the elements arranged into rows and Elements with similar
columns according to their physical and chemical properties were grouped in
properties. the same column.
Mendeleev noticed that melting point is one property
that shows a repeating pattern.
3 4 5 6 7 8 9 10
Row 2 Li Be B C N O F Ne
Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon
11 12 13 14 15 16 17 18
Row 3 Na Mg Al Si P S Cl Ar
Sodium Magnesium Aluminum Silicon Phosphorus Sulfur Chlorine Argon
214