Page 231 - Science Course 1 (Book 1)
P. 231
Mo6-L5a: What is The Periodic Table?
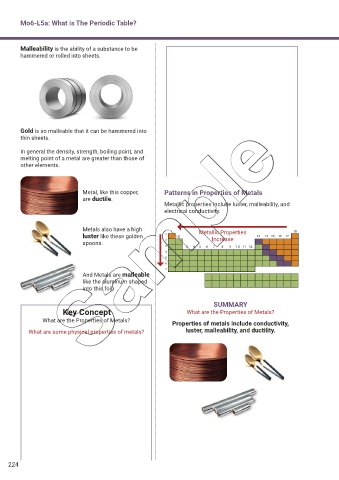
Malleability is the ability of a substance to be
hammered or rolled into sheets.
Gold is so malleable that it can be hammered into
thin sheets.
In general the density, strength, boiling point, and
melting point of a metal are greater than those of
other elements.
Metal, like this copper, Patterns in Properties of Metals
are ductile.
Metallic properties include luster, malleability, and
electrical conductivity.
Metals also have a high Metallic Properties
luster like these golden Increase
spoons.
And Metals are malleable
like the aluminum shaped
into this foil.
SUMMARY
Key Concept What are the Properties of Metals?
What are the Properties of Metals? Properties of metals include conductivity,
What are some physical properties of metals? luster, malleability, and ductility.
224