Page 232 - Science Course 1 (Book 1)
P. 232
Mo6-L5b: How are Metals Grouped?
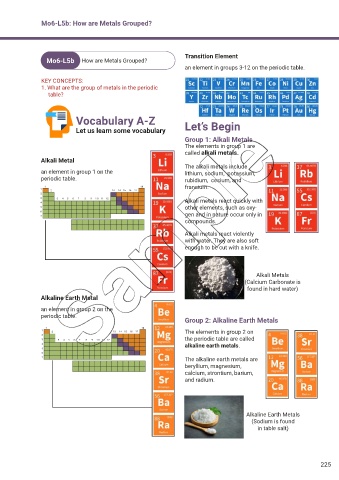
Transition Element
Mo6-L5b How are Metals Grouped?
an element in groups 3-12 on the periodic table.
KEY CONCEPTS:
1. What are the group of metals in the periodic
table?
Vocabulary A-Z
Let us learn some vocabulary Let’s Begin
Group 1: Alkali Metals
The elements in group 1 are
called alkali metals.
Alkali Metal
The alkali metals include
an element in group 1 on the lithium, sodium, potassium,
periodic table. rubidium, cesium, and
francium.
Alkali metals react quickly with
other elements, such as oxy-
gen and in nature occur only in
compounds.
Alkali metals react violently
with water. They are also soft
enough to be cut with a knife.
Alkali Metals
(Calcium Carbonate is
found in hard water)
Alkaline Earth Metal
an element in group 2 on the
periodic table.
Group 2: Alkaline Earth Metals
The elements in group 2 on
the periodic table are called
alkaline earth metals.
The alkaline earth metals are
beryllium, magnesium,
calcium, strontium, barium,
and radium.
Alkaline Earth Metals
(Sodium is found
in table salt)
225