Page 233 - Science Course 1 (Book 1)
P. 233
Mo6-L5b: How are Metals Grouped?
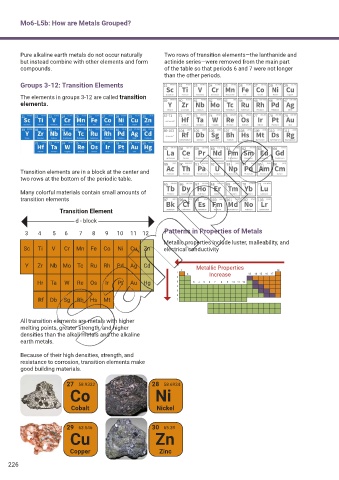
Pure alkaline earth metals do not occur naturally Two rows of transition elements—the lanthanide and
but instead combine with other elements and form actinide series—were removed from the main part
compounds. of the table so that periods 6 and 7 were not longer
than the other periods.
Groups 3-12: Transition Elements
The elements in groups 3-12 are called transition
elements.
Transition elements are in a block at the center and
two rows at the bottom of the periodic table.
Many colorful materials contain small amounts of
transition elements
Transition Element
d - block
3 4 5 6 7 8 9 10 11 12 Patterns in Properties of Metals
Metallic properties include luster, malleability, and
Sc Ti V Cr Mn Fe Co Ni Cu Zn electrical conductivity
Y Zr Nb Mo Tc Ru Rh Pd Ag Cd Metallic Properties
Increase
Hr Ta W Re Os Ir Pt Au Hg
Rf Db Sg Bh Hs Mt
All transition elements are metals with higher
melting points, greater strength, and higher
densities than the alkali metals and the alkaline
earth metals.
Because of their high densities, strength, and
resistance to corrosion, transition elements make
good building materials.
27 58.9332 28 58.6934
Co Ni
Cobalt Nickel
29 63.546 30 65.39
Cu Zn
Copper Zinc
226