Page 181 - Science Course 1 (Book 1)
P. 181
Mo5-L4a: How are Boyle's Law and Charles Law Related?
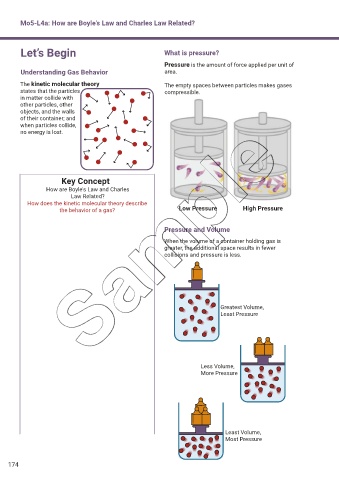
Let’s Begin What is pressure?
Pressure is the amount of force applied per unit of
Understanding Gas Behavior area.
The kinetic molecular theory The empty spaces between particles makes gases
states that the particles compressible.
in matter collide with
other particles, other
objects, and the walls
of their container; and
when particles collide,
no energy is lost.
Key Concept
How are Boyle's Law and Charles
Law Related?
How does the kinetic molecular theory describe
the behavior of a gas? Low Pressure High Pressure
Pressure and Volume
When the volume of a container holding gas is
greater, the additional space results in fewer
collisions and pressure is less.
Greatest Volume,
Least Pressure
Less Volume,
More Pressure
Least Volume,
Most Pressure
174