Page 183 - Science Course 1 (Book 1)
P. 183
Mo5-L4a: How are Boyle's Law and Charles Law Related?
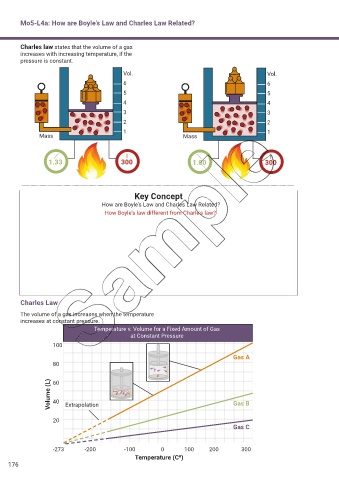
Charles law states that the volume of a gas
increases with increasing temperature, if the
pressure is constant.
Vol. Vol.
6 6
5 5
4 4
3 3
2 2
1 1
Mass Mass
1.33 300 1.00 300
Key Concept
How are Boyle's Law and Charles Law Related?
How Boyle's law different from Charle's law?
Charles Law
The volume of a gas increases when the temperature
increases at constant pressure.
Temperature v. Volume for a Fixed Amount of Gas
at Constant Pressure
100
Gas A
80
Volume (L) 60 Extrapolation Gas B
40
20
Gas C
-273 -200 -100 0 100 200 300
Temperature (C0)
176