Page 182 - Science Course 1 (Book 1)
P. 182
Mo5-L4a: How are Boyle's Law and Charles Law Related?
Boyle’s Law
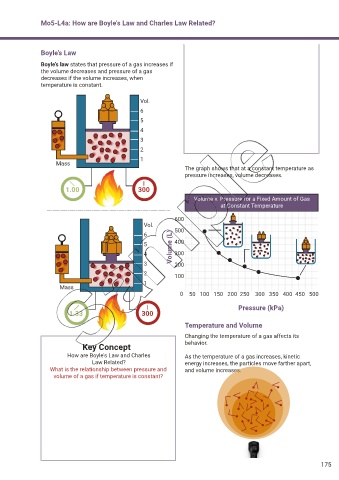
Boyle’s law states that pressure of a gas increases if
the volume decreases and pressure of a gas
decreases if the volume increases, when
temperature is constant.
Vol.
6
5
4
3
2
1
Mass
The graph shows that at a constant temperature as
pressure increases, volume decreases.
1.00 300
Volume v. Pressure for a Fixed Amount of Gas
at Constant Temperature
600
Vol.
6 500
5 Volume (L) 400
4 300
3 200
2 100
1
Mass
0 50 100 150 200 250 300 350 400 450 500
Pressure (kPa)
1.33 300
Temperature and Volume
Changing the temperature of a gas affects its
Key Concept behavior.
How are Boyle's Law and Charles As the temperature of a gas increases, kinetic
Law Related? energy increases, the particles move farther apart,
What is the relationship between pressure and and volume increases.
volume of a gas if temperature is constant?
175