Page 223 - Science Course 3 (Book 1)
P. 223
Mo6-L1b: Metals and Non-Metals
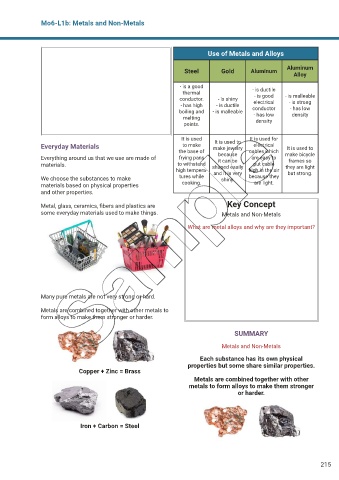
Use of Metals and Alloys
Steel Gold Aluminum Aluminum
Alloy
- is a good - is ductile
thermal - is good - is malleable
conductor. - is shiny electrical - is strong
- has high - is ductile conductor - has low
boiling and - is malleable - has low density
melting density
points.
It is used It is used to It is used for
Everyday Materials to make make jewelry electrical It is used to
the base of cables which
Everything around us that we use are made of frying pans because are easy to make bicycle
it can be
frames so
materials. to withstand shaped easily put cable they are light
high tempera- and it is very high in the air but strong.
We choose the substances to make tures while shiny. because they
materials based on physical properties cooking. are light.
and other properties.
Metal, glass, ceramics, fibers and plastics are Key Concept
some everyday materials used to make things. Metals and Non-Metals
What are metal alloys and why are they important?
Many pure metals are not very strong or hard.
Metals are combined together with other metals to
form alloys to make them stronger or harder.
SUMMARY
Metals and Non-Metals
Each substance has its own physical
properties but some share similar properties.
Copper + Zinc = Brass
Metals are combined together with other
metals to form alloys to make them stronger
or harder.
Iron + Carbon = Steel
215