Page 256 - Science Course 3 (Book 1)
P. 256
Chemical Reactions: Change in Energy Mo7-L3a: Why is There a Change in Energy?
Lesson 3
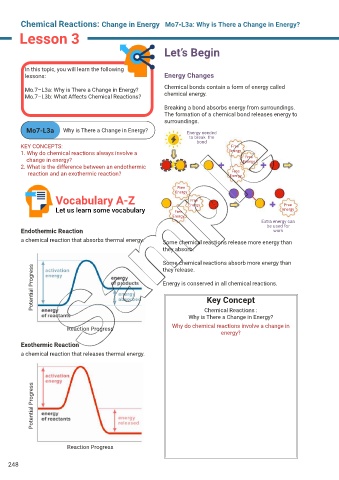
Let’s Begin
In this topic, you will learn the following
lessons: Energy Changes
Mo.7–L3a: Why is There a Change in Energy? Chemical bonds contain a form of energy called
Mo.7–L3b: What Affects Chemical Reactions? chemical energy.
Breaking a bond absorbs energy from surroundings.
The formation of a chemical bond releases energy to
surroundings.
Mo7-L3a Why is There a Change in Energy? Energy needed
to break the
bond
KEY CONCEPTS: Free
1. Why do chemical reactions always involve a Energy
Free
change in energy? Energy
2. What is the difference between an endothermic
Free
reaction and an exothermic reaction? Energy
Free
Energy
Vocabulary A-Z Free
Free
Let us learn some vocabulary Free Energy Energy
Energy
Extra energy can
be used for
Endothermic Reaction work
a chemical reaction that absorbs thermal energy. Some chemical reactions release more energy than
they absorb.
Some chemical reactions absorb more energy than
Potential Progress Energy is conserved in all chemical reactions.
they release.
Key Concept
Chemical Reactions :
Why is There a Change in Energy?
Reaction Progress Why do chemical reactions involve a change in
energy?
Exothermic Reaction
a chemical reaction that releases thermal energy.
Potential Progress
Reaction Progress
248