Page 258 - Science Course 3 (Book 1)
P. 258
Mo7-L3b: What Affects Chemical Reactions?
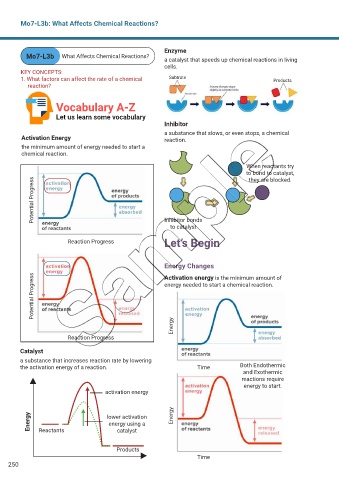
Enzyme
Mo7-L3b What Affects Chemical Reactions? a catalyst that speeds up chemical reactions in living
cells.
KEY CONCEPTS:
1. What factors can affect the rate of a chemical Subtrate Products
reaction?
Vocabulary A-Z
Let us learn some vocabulary
Inhibitor
a substance that slows, or even stops, a chemical
Activation Energy reaction.
the minimum amount of energy needed to start a
chemical reaction.
When reactants try
to bond to catalyst,
they are blocked.
Potential Progress
Inhibitor bonds
to catalyst
Reaction Progress Let’s Begin
Energy Changes
Potential Progress energy needed to start a chemical reaction.
Activation energy is the minimum amount of
Energy
Reaction Progress
Catalyst
a substance that increases reaction rate by lowering
the activation energy of a reaction. Time Both Endothermic
and Exothermic
reactions require
energy to start.
activation energy
Energy Reactants lower activation Energy
energy using a
catalyst
Products
Time
250