Page 257 - Science Course 3 (Book 1)
P. 257
Mo7-L3a: Why There’s a Change in Energy?
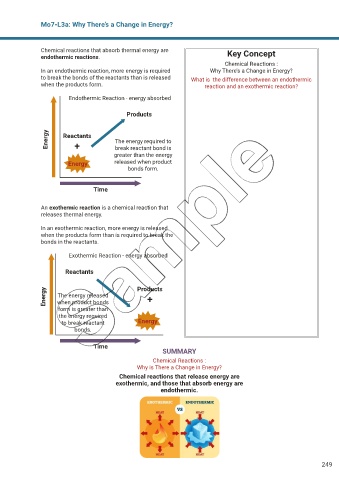
Chemical reactions that absorb thermal energy are Key Concept
endothermic reactions.
Chemical Reactions :
In an endothermic reaction, more energy is required Why There’s a Change in Energy?
to break the bonds of the reactants than is released What is the difference between an endothermic
when the products form. reaction and an exothermic reaction?
Endothermic Reaction - energy absorbed
Products
Energy Reactants The energy required to
+
break reactant bond is
greater than the energy
Energy released when product
bonds form.
Time
An exothermic reaction is a chemical reaction that
releases thermal energy.
In an exothermic reaction, more energy is released
when the products form than is required to break the
bonds in the reactants.
Exothermic Reaction - energy absorbed
Reactants
Energy The energy released Products
+
when product bonds
form is greater than
the energy required
to break reactant Energy
bonds.
Time
SUMMARY
Chemical Reactions :
Why is There a Change in Energy?
Chemical reactions that release energy are
exothermic, and those that absorb energy are
endothermic.
249