Page 259 - Science Course 3 (Book 1)
P. 259
Mo7-L3b: What Affects Chemical Reactions?
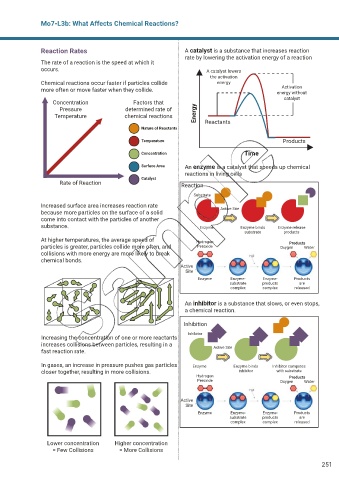
Reaction Rates A catalyst is a substance that increases reaction
rate by lowering the activation energy of a reaction
The rate of a reaction is the speed at which it
occurs. A catalyst lowers
the activation
Chemical reactions occur faster if particles collide energy
Activation
more often or move faster when they collide. energy without
catalyst
Concentration Factors that
Pressure determined rate of
Temperature chemical reactions Energy Reactants
Nature of Reactants
Temperature Products
Concentration Time
Surface Area An enzyme is a catalyst that speeds up chemical
reactions in living cells
Catalyst
Rate of Reaction Reaction
Substrate
Increased surface area increases reaction rate Active Site
because more particles on the surface of a solid
come into contact with the particles of another
substance. Enzyme Enzyme binds Enzyme release
substrate products
At higher temperatures, the average speed of Hydrogen Products
particles is greater, particles collide more often, and Peroxide Oxygen Water
collisions with more energy are more likely to break
chemical bonds.
Active
Site
Enzyme Enzyme- Enzyme- Products
substrate products are
complex complex released
An inhibitor is a substance that slows, or even stops,
a chemical reaction.
Inhibition
Inhibitor
Increasing the concentration of one or more reactants
increases collisions between particles, resulting in a Active Site
fast reaction rate.
In gases, an increase in pressure pushes gas particles Enzyme Enzyme binds Inhibitor competes
closer together, resulting in more collisions. inhibitor with substrate
Hydrogen Products
Peroxide Oxygen Water
Active
Site
Enzyme Enzyme- Enzyme- Products
substrate products are
complex complex released
Lower concentration Higher concentration
= Few Collisions = More Collisions
251