Page 123 - Science Course 3 (Book 2)
P. 123
Mo11-L3b: How to Determine Absolute Ages?
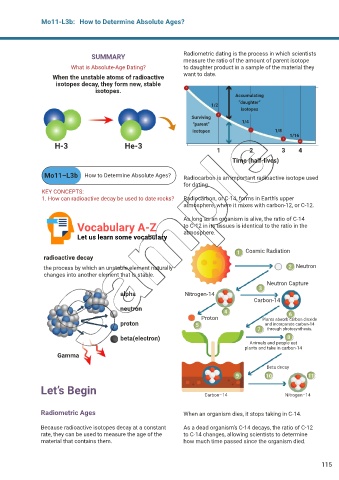
SUMMARY Radiometric dating is the process in which scientists
measure the ratio of the amount of parent isotope
What is Absolute-Age Dating? to daughter product in a sample of the material they
When the unstable atoms of radioactive want to date.
isotopes decay, they form new, stable
isotopes.
Accumulating
“daughter”
1/2
isotopes
Surviving
1/4
“parent”
isotopes 1/8
1/16
H-3 He-3
1 2 3 4
Time (half-lives)
Mo11–L3b How to Determine Absolute Ages? Radiocarbon is an important radioactive isotope used
for dating.
KEY CONCEPTS:
1. How can radioactive decay be used to date rocks? Radiocarbon, or C-14, forms in Earth’s upper
atmosphere, where it mixes with carbon-12, or C-12.
As long as an organism is alive, the ratio of C-14
Vocabulary A-Z to C-12 in its tissues is identical to the ratio in the
atmosphere.
Let us learn some vocabulary
Cosmic Radiation
radioactive decay
the process by which an unstable element naturally Neutron
changes into another element that is stable.
Neutron Capture
alpha Nitrogen-14
Carbon-14
neutron
Proton Plants absorb carbon dioxide
proton and incorporate carbon-14
through photosynthesis.
beta(electron)
Animals and people eat
plants and take in carbon-14
Gamma
Beta decay
Let’s Begin
Carbon–14 Nitrogen–14
Radiometric Ages When an organism dies, it stops taking in C-14.
Because radioactive isotopes decay at a constant As a dead organism’s C-14 decays, the ratio of C-12
rate, they can be used to measure the age of the to C-14 changes, allowing scientists to determine
material that contains them. how much time passed since the organism died.
115