Page 169 - Science Course 1 (Book 1)
P. 169
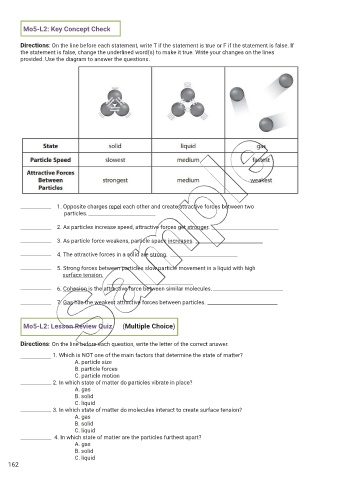
Mo5-L2: Key Concept Check
Directions: On the line before each statement, write T if the statement is true or F if the statement is false. If
the statement is false, change the underlined word(s) to make it true. Write your changes on the lines
provided. Use the diagram to answer the questions.
1. Opposite charges repel each other and create attractive forces between two
particles.
2. As particles increase speed, attractive forces get stronger.
3. As particle force weakens, particle space increases.
4. The attractive forces in a solid are strong.
5. Strong forces between particles slow particle movement in a liquid with high
surface tension.
6. Cohesion is the attractive force between similar molecules.
7. Gas has the weakest attractive forces between particles.
Mo5-L2: Lesson Review Quiz (Multiple Choice)
Directions: On the line before each question, write the letter of the correct answer.
1. Which is NOT one of the main factors that determine the state of matter?
A. particle size
B. particle forces
C. particle motion
2. In which state of matter do particles vibrate in place?
A. gas
B. solid
C. liquid
3. In which state of matter do molecules interact to create surface tension?
A. gas
B. solid
C. liquid
4. In which state of matter are the particles furthest apart?
A. gas
B. solid
C. liquid
162