Page 171 - Science Course 1 (Book 1)
P. 171
Matter: Changes in State Mo5-L3a: What is the Effect of Temperature
and Energy on Matter?
Lesson 3
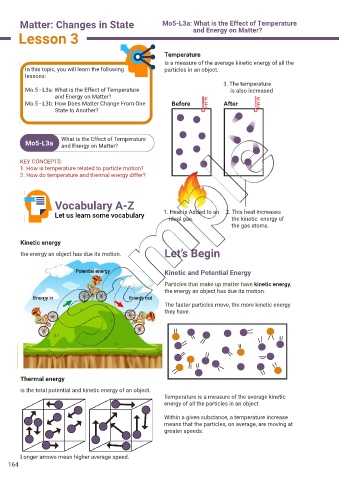
Temperature
is a measure of the average kinetic energy of all the
In this topic, you will learn the following particles in an object.
lessons:
3. The temperature
Mo.5 - L3a: What is the Effect of Temperature is also increased
and Energy on Matter?
Mo.5 - L3b: How Does Matter Change From One Before After
State to Another?
Mo5-L3a What is the Effect of Temperature
and Energy on Matter?
KEY CONCEPTS:
1. How is temperature related to particle motion?
2. How do temperature and thermal energy differ?
Vocabulary A-Z
Let us learn some vocabulary 1. Heat is Added to an 2. This heat increases
ideal gas.
the kinetic energy of
the gas atoms.
Kinetic energy
the energy an object has due its motion. Let’s Begin
Kinetic and Potential Energy
Particles that make up matter have kinetic energy,
the energy an object has due its motion.
The faster particles move, the more kinetic energy
they have.
Thermal energy
is the total potential and kinetic energy of an object.
Temperature is a measure of the average kinetic
energy of all the particles in an object.
Within a given substance, a temperature increase
means that the particles, on average, are moving at
greater speeds.
Longer arrows mean higher average speed.
164