Page 189 - Science Course 1 (Book 1)
P. 189
Introduction to The Atom Mo6-L1a: How did the Atomic Theory Start?
Lesson 1
Let’s Begin
In this topic, you will learn the following Early Ideas About Matter
lessons:
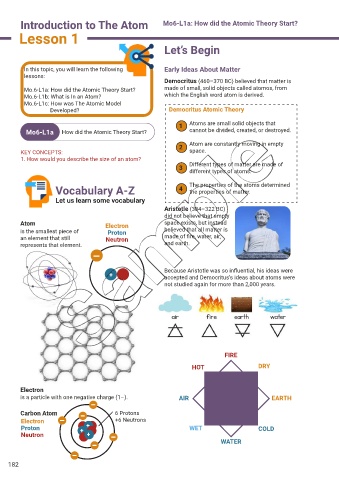
Democritus (460–370 BC) believed that matter is
Mo.6-L1a: How did the Atomic Theory Start? made of small, solid objects called atomos, from
Mo.6-L1b: What is In an Atom? which the English word atom is derived.
Mo.6-L1c: How was The Atomic Model
Developed? Democritus Atomic Theory
1 Atoms are small solid objects that
Mo6-L1a How did the Atomic Theory Start? cannot be divided, created, or destroyed.
2 Atom are constantly moving in empty
KEY CONCEPTS: space.
1. How would you describe the size of an atom?
3 Different types of matter are made of
different types of atoms.
Vocabulary A-Z 4 The properties of the atoms determined
the properties of matter.
Let us learn some vocabulary
Aristotle (384–322 BC)
did not believe that empty
Atom Electron space exists, but instead
is the smallest piece of Proton believed that all matter is
an element that still Neutron made of fire, water, air,
represents that element. and earth.
Because Aristotle was so influential, his ideas were
+ accepted and Democritus’s ideas about atoms were
not studied again for more than 2,000 years.
FIRE
HOT DRY
Electron
is a particle with one negative charge (1–). AIR EARTH
Carbon Atom 6 Protons
Electron +6 Neutrons
Proton + + WET COLD
Neutron +
WATER
182