Page 192 - Science Course 1 (Book 1)
P. 192
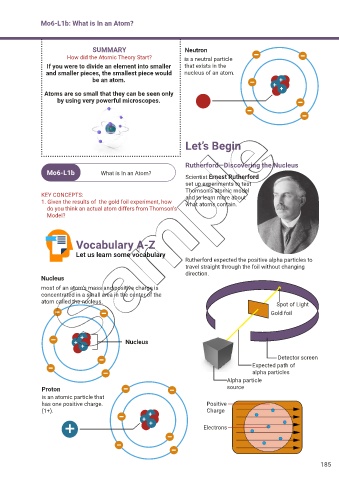
Mo6-L1b: What is In an Atom?
SUMMARY Neutron
How did the Atomic Theory Start? is a neutral particle
If you were to divide an element into smaller that exists in the
and smaller pieces, the smallest piece would nucleus of an atom.
be an atom. +
+ +
Atoms are so small that they can be seen only
by using very powerful microscopes.
Let’s Begin
Rutherford—Discovering the Nucleus
Mo6-L1b What is In an Atom?
Scientist Ernest Rutherford
set up experiments to test
Thomson’s atomic model
KEY CONCEPTS: and to learn more about
1. Given the results of the gold foil experiment, how what atoms contain.
do you think an actual atom differs from Thomson’s
Model?
Vocabulary A-Z
Let us learn some vocabulary
Rutherford expected the positive alpha particles to
travel straight through the foil without changing
direction.
Nucleus
most of an atom’s mass and positive charge is
concentrated in a small area in the center of the
atom called the nucleus. Spot of Light
Gold foil
+
+ Nucleus
+
Detector screen
Expected path of
alpha particles
Alpha particle
Proton source
is an atomic particle that
has one positive charge. Positive
(1+). + Charge
+
+
Electrons
185