Page 193 - Science Course 1 (Book 1)
P. 193
Mo6-L1b: What is In an Atom?
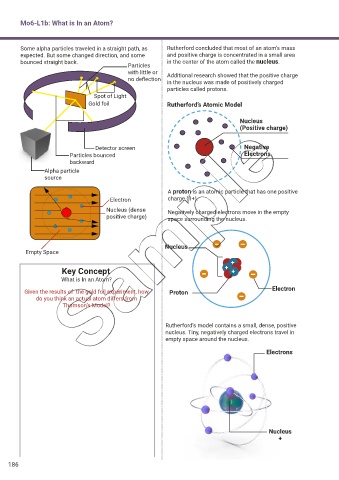
Some alpha particles traveled in a straight path, as Rutherford concluded that most of an atom’s mass
expected. But some changed direction, and some and positive charge is concentrated in a small area
bounced straight back. in the center of the atom called the nucleus.
Particles
with little or Additional research showed that the positive charge
no deflection
in the nucleus was made of positively charged
particles called protons.
Spot of Light
Gold foil Rutherford’s Atomic Model
Nucleus
(Positive charge)
Detector screen Negative
Particles bounced Electrons
backward
Alpha particle
source
A proton is an atomic particle that has one positive
Electron charge (1+).
Nucleus (dense Negatively charged electrons move in the empty
positive charge) space surrounding the nucleus.
Nucleus
Empty Space
+
Key Concept + +
What is In an Atom?
Given the results of the gold foil experiment, how Proton Electron
do you think an actual atom differs from
Thomson’s Model?
Rutherford’s model contains a small, dense, positive
nucleus. Tiny, negatively charged electrons travel in
empty space around the nucleus.
Electrons
Nucleus
+
186