Page 194 - Science Course 1 (Book 1)
P. 194
Mo6-L1c: How was The Atomic Model Developed?
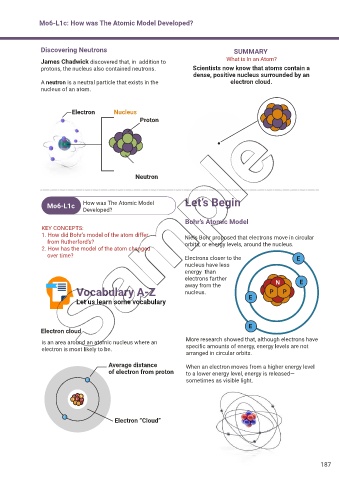
Discovering Neutrons SUMMARY
James Chadwick discovered that, in addition to What is In an Atom?
protons, the nucleus also contained neutrons. Scientists now know that atoms contain a
dense, positive nucleus surrounded by an
A neutron is a neutral particle that exists in the electron cloud.
nucleus of an atom.
Electron Nucleus
Proton
Neutron
Mo6-L1c How was The Atomic Model Let’s Begin
Developed?
Bohr’s Atomic Model
KEY CONCEPTS:
1. How did Bohr’s model of the atom differ Niels Bohr proposed that electrons move in circular
from Rutherford’s? orbits, or energy levels, around the nucleus.
2. How has the model of the atom changed
over time? Electrons closer to the E
nucleus have less
energy than
electrons farther N E
Vocabulary A-Z away from the P P
nucleus.
Let us learn some vocabulary E
E
Electron cloud
is an area around an atomic nucleus where an More research showed that, although electrons have
electron is most likely to be. specific amounts of energy, energy levels are not
arranged in circular orbits.
Average distance When an electron moves from a higher energy level
of electron from proton to a lower energy level, energy is released—
E sometimes as visible light.
P P N
N P P
N N P
E
Electron “Cloud”
187