Page 195 - Science Course 1 (Book 1)
P. 195
Mo6-L1c: How was The Atomic Model Developed?
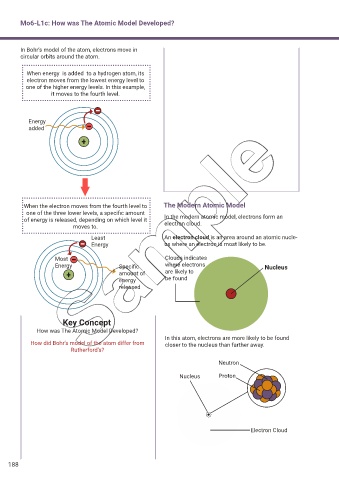
In Bohr’s model of the atom, electrons move in
circular orbits around the atom.
When energy is added to a hydrogen atom, its
electron moves from the lowest energy level to
one of the higher energy levels. In this example,
it moves to the fourth level.
Energy
added
When the electron moves from the fourth level to The Modern Atomic Model
one of the three lower levels, a specific amount
of energy is released, depending on which level it In the modern atomic model, electrons form an
electron cloud.
moves to.
Least An electron cloud is an area around an atomic nucle-
Energy us where an electron is most likely to be.
Most Clouds indicates
Energy Specific where electrons Nucleus
amount of are likely to
energy be found
released
Key Concept
How was The Atomic Model Developed?
In this atom, electrons are more likely to be found
How did Bohr’s model of the atom differ from closer to the nucleus than farther away.
Rutherford’s?
Neutron
Nucleus Proton
Electron Cloud
188