Page 201 - Science Course 1 (Book 1)
P. 201
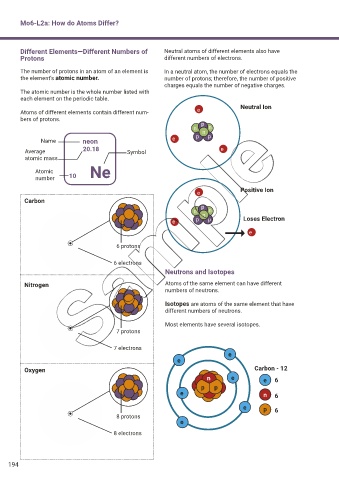
Mo6-L2a: How do Atoms Differ?
Different Elements—Different Numbers of Neutral atoms of different elements also have
Protons different numbers of electrons.
The number of protons in an atom of an element is In a neutral atom, the number of electrons equals the
the element’s atomic number. number of protons; therefore, the number of positive
charges equals the number of negative charges.
The atomic number is the whole number listed with
each element on the periodic table.
Neutral Ion
Atoms of different elements contain different num- e
bers of protons.
n p n n
Name neon e p p
Average 20.18 Symbol e
atomic mass
Atomic Ne
number 10
e Positive Ion
Carbon
p
n n n
e p p Loses Electron
e
6 protons
6 electrons
Neutrons and Isotopes
Nitrogen Atoms of the same element can have different
numbers of neutrons.
Isotopes are atoms of the same element that have
different numbers of neutrons.
Most elements have several isotopes.
7 protons
7 electrons
e
e
Oxygen Carbon - 12
n e e 6
p p
e n 6
e p 6
8 protons
e
8 electrons
194