Page 205 - Science Course 1 (Book 1)
P. 205
Mo6-L2b: What is a Nuclear Decay?
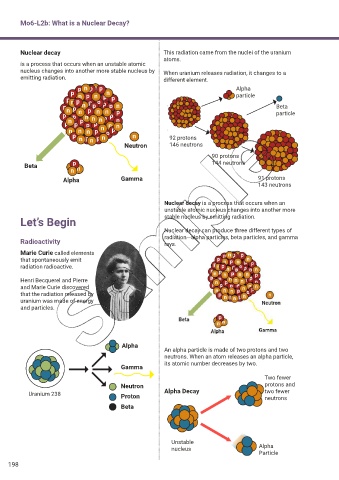
Nuclear decay This radiation came from the nuclei of the uranium
atoms.
is a process that occurs when an unstable atomic
nucleus changes into another more stable nucleus by When uranium releases radiation, it changes to a
emitting radiation. different element.
p n p n p Alpha
p n particle
p n p n p n p p
p p
p n p n p p p p n Beta
p p n n p n n n p n p p particle
p
p n p p p p n n
n n n p n p p
p n p n p n n 92 protons
Neutron 146 neutrons
90 protons
Beta n p 144 neutrons
n n
Alpha Gamma 91 protons
143 neutrons
Nuclear decay is a process that occurs when an
unstable atomic nucleus changes into another more
Let’s Begin stable nucleus by emitting radiation.
Nuclear decay can produce three different types of
radiation—alpha particles, beta particles, and gamma
Radioactivity rays.
Marie Curie called elements
that spontaneously emit
radiation radioactive.
Henri Becquerel and Pierre
and Marie Curie discovered
that the radiation released by
uranium was made of energy
and particles.
Alpha
An alpha particle is made of two protons and two
neutrons. When an atom releases an alpha particle,
its atomic number decreases by two.
Gamma
Two fewer
Neutron protons and
Uranium 238 Proton Alpha Decay two fewer
neutrons
Beta
Unstable
nucleus Alpha
Particle
198