Page 202 - Science Course 1 (Book 1)
P. 202
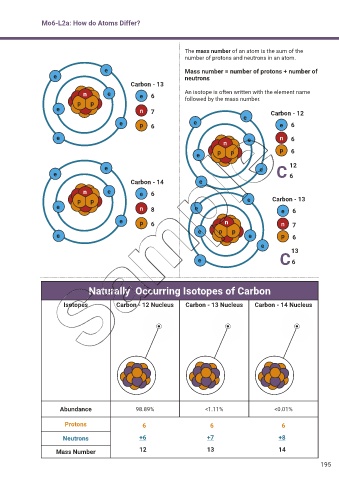
Mo6-L2a: How do Atoms Differ?
The mass number of an atom is the sum of the
number of protons and neutrons in an atom.
e Mass number = number of protons + number of
e neutrons
Carbon - 13
n e e 6 An isotope is often written with the element name
p p followed by the mass number.
e n 7 Carbon - 12
e p 6 e e e 6
e e n 6
n
e p p p 6
e e 12
e C 6
Carbon - 14 e
n e e 6
p p e Carbon - 13
e n 8 e e 6
e p 6 n n 7
e p p
e e p 6
e
13
e C 6
Naturally Occurring Isotopes of Carbon
Isotopes Carbon - 12 Nucleus Carbon - 13 Nucleus Carbon - 14 Nucleus
Abundance 98.89% <1.11% <0.01%
Protons 6 6 6
Neutrons +6 +7 +8
Mass Number 12 13 14
195