Page 204 - Science Course 1 (Book 1)
P. 204
Mo6-L2b: What is a Nuclear Decay?
SUMMARY
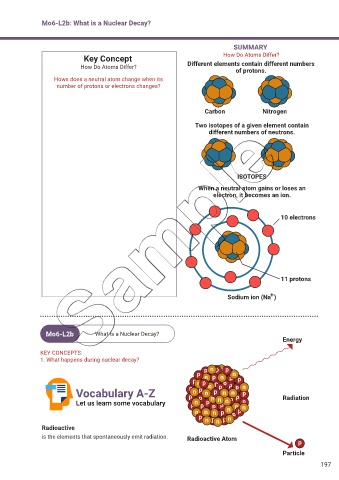
Key Concept How Do Atoms Differ?
How Do Atoms Differ? Different elements contain different numbers
of protons.
Hows does a neutral atom change when its
number of protons or electrons changes?
Carbon Nitrogen
Two isotopes of a given element contain
different numbers of neutrons.
ISOTOPES
When a neutral atom gains or loses an
electron, it becomes an ion.
10 electrons
11 protons
+
Sodium ion (Na )
Mo6-L2b What is a Nuclear Decay?
Energy
KEY CONCEPTS:
1. What happens during nuclear decay?
p n p n p
p n
p p
p n p n n p n p
p p
Vocabulary A-Z p n p n p p n n p n
p
p
p
p
Let us learn some vocabulary p n p p p n p p n p n p n n Radiation
n n n p n p p
p n p n p n
Radioactive
is the elements that spontaneously emit radiation. Radioactive Atom
p
Particle
197