Page 203 - Science Course 1 (Book 1)
P. 203
Mo6-L2a: How do Atoms Differ?
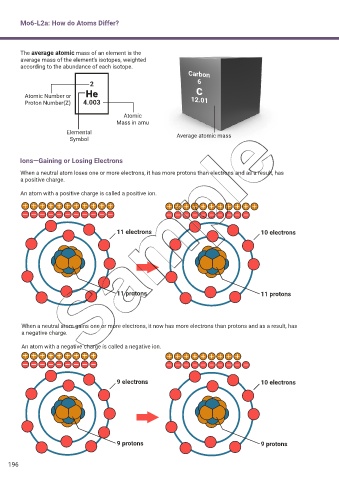
The average atomic mass of an element is the
average mass of the element’s isotopes, weighted
according to the abundance of each isotope.
Carbon
2 6
C
Atomic Number or He 12.01
Proton Number(Z) 4.003
Atomic
Mass in amu
Elemental Average atomic mass
Symbol
Ions—Gaining or Losing Electrons
When a neutral atom loses one or more electrons, it has more protons than electrons and as a result, has
a positive charge.
An atom with a positive charge is called a positive ion.
11 electrons 10 electrons
11 protons 11 protons
When a neutral atom gains one or more electrons, it now has more electrons than protons and as a result, has
a negative charge.
An atom with a negative charge is called a negative ion.
9 electrons 10 electrons
9 protons 9 protons
196