Page 211 - Science Course 1 (Book 1)
P. 211
Mo6-L3a: What are the Parts of an Atom?
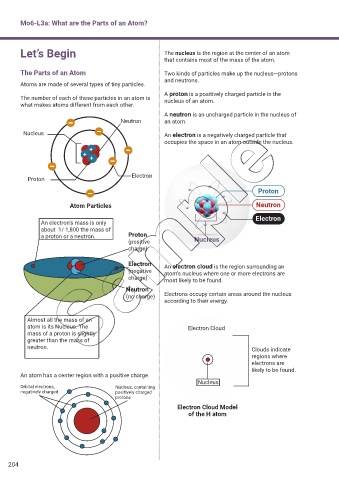
Let’s Begin The nucleus is the region at the center of an atom
that contains most of the mass of the atom.
The Parts of an Atom Two kinds of particles make up the nucleus—protons
and neutrons.
Atoms are made of several types of tiny particles.
A proton is a positively charged particle in the
The number of each of these particles in an atom is nucleus of an atom.
what makes atoms different from each other.
A neutron is an uncharged particle in the nucleus of
Neutron an atom.
Nucleus An electron is a negatively charged particle that
occupies the space in an atom outside the nucleus.
+
+
+
Electron
Proton
Proton
Atom Particles Neutron
Electron
An electron’s mass is only
about 1/ 1,800 the mass of
a proton or a neutron. Proton Nucleus
(positive
charge)
Electron An electron cloud is the region surrounding an
(negative atom’s nucleus where one or more electrons are
charge) most likely to be found.
Neutron
(no charge) Electrons occupy certain areas around the nucleus
according to their energy.
Almost all the mass of an
atom is its Nucleus. The Electron Cloud
mass of a proton is slightly
greater than the mass of
neutron. Clouds indicate
regions where
electrons are
likely to be found.
An atom has a center region with a positive charge.
Nucleus
Orbital electrons, Nucleus, containing
negatively charged positively charged
protons
Electron Cloud Model
of the H atom
204