Page 216 - Science Course 1 (Book 1)
P. 216
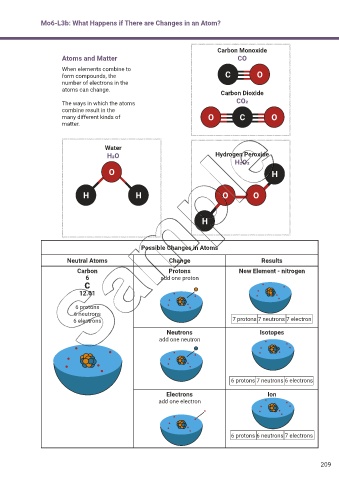
Mo6-L3b: What Happens if There are Changes in an Atom?
Carbon Monoxide
Atoms and Matter CO
When elements combine to
form compounds, the C O
number of electrons in the
atoms can change. Carbon Dioxide
The ways in which the atoms CO2
combine result in the
many different kinds of O C O
matter.
Water
H2O Hydrogen Peroxide
H2O2
O H
H H O O
H
Possible Changes in Atoms
Neutral Atoms Change Results
Carbon Protons New Element - nitrogen
6 add one proton
C
12.01
6 protons
6 neutrons
6 electrons 7 protons 7 neutrons 7 electron
Neutrons Isotopes
add one neutron
6 protons 7 neutrons 6 electrons
Electrons Ion
add one electron
6 protons 6 neutrons 7 electrons
209