Page 212 - Science Course 1 (Book 1)
P. 212
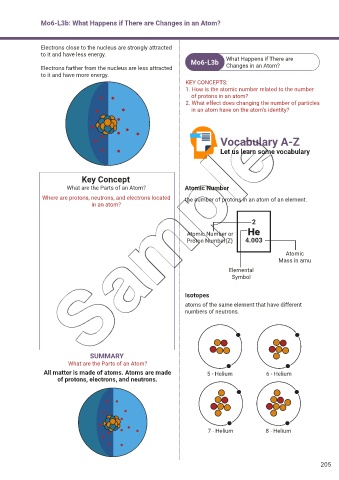
Mo6-L3b: What Happens if There are Changes in an Atom?
Electrons close to the nucleus are strongly attracted
to it and have less energy.
Mo6-L3b What Happens if There are
Electrons farther from the nucleus are less attracted Changes in an Atom?
to it and have more energy.
KEY CONCEPTS:
1. How is the atomic number related to the number
of protons in an atom?
2. What effect does changing the number of particles
in an atom have on the atom’s identity?
Vocabulary A-Z
Let us learn some vocabulary
Key Concept
What are the Parts of an Atom? Atomic Number
Where are protons, neutrons, and electrons located the number of protons in an atom of an element.
in an atom?
2
Atomic Number or He
Proton Number(Z) 4.003
Atomic
Mass in amu
Elemental
Symbol
Isotopes
atoms of the same element that have different
numbers of neutrons.
SUMMARY
What are the Parts of an Atom?
All matter is made of atoms. Atoms are made 5 - Helium 6 - Helium
of protons, electrons, and neutrons.
7 - Helium 8 - Helium
205