Page 213 - Science Course 1 (Book 1)
P. 213
Mo6-L3b: What Happens if There are Changes in an Atom?
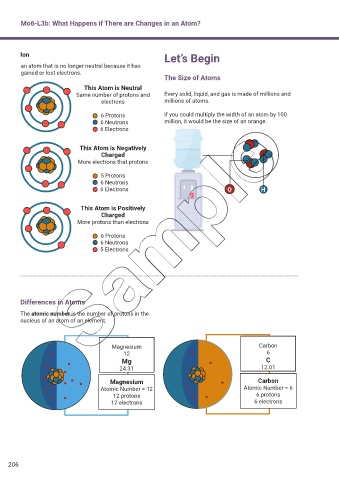
Ion Let’s Begin
an atom that is no longer neutral because it has
gained or lost electrons.
The Size of Atoms
This Atom is Neutral
Same number of protons and Every solid, liquid, and gas is made of millions and
electrons millions of atoms.
6 Protons If you could multiply the width of an atom by 100
6 Neutrons million, it would be the size of an orange.
6 Electrons
This Atom is Negatively
Charged
More electrons that protons
5 Protons
6 Neutrons
6 Electrons O H
This Atom is Positively
Charged
More protons than electrons
6 Protons
6 Neutrons
5 Electrons
Differences in Atoms
The atomic number is the number of protons in the
nucleus of an atom of an element.
Magnesium Carbon
12 6
Mg C
24.31 12.01
Magnesium Carbon
Atomic Number = 12 Atomic Number = 6
12 protons 6 protons
12 electrons 6 electrons
206