Page 215 - Science Course 1 (Book 1)
P. 215
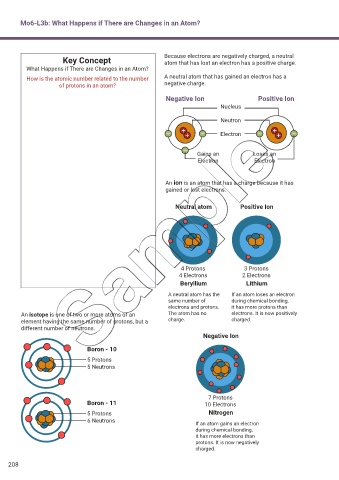
Mo6-L3b: What Happens if There are Changes in an Atom?
Key Concept Because electrons are negatively charged, a neutral
atom that has lost an electron has a positive charge.
What Happens if There are Changes in an Atom?
How is the atomic number related to the number A neutral atom that has gained an electron has a
of protons in an atom? negative charge.
Negative Ion Positive Ion
Nucleus
Neutron
Electron
Gains an Loses an
Electron Electron
An ion is an atom that has a charge because it has
gained or lost electrons.
Neutral atom Positive Ion
4 Protons 3 Protons
4 Electrons 2 Electrons
Beryllium Lithium
A neutral atom has the If an atom loses an electron
same number of during chemical bonding,
electrons and protons. it has more protons than
An isotope is one of two or more atoms of an The atom has no electrons. It is now positively
element having the same number of protons, but a charge. charged.
different number of neutrons.
Negative Ion
Boron - 10
5 Protons
5 Neutrons
7 Protons
Boron - 11 10 Electrons
5 Protons Nitrogen
6 Neutrons If an atom gains an electron
during chemical bonding,
it has more electrons than
protons. It is now negatively
charged.
208