Page 117 - Science Course 2 (Book 1)
P. 117
pH of a Solution Mo4-L3a: What are Acids and Bases?
Lesson 3
Let’s Begin
In this topic, you will learn the following
lessons: What are acids and bases?
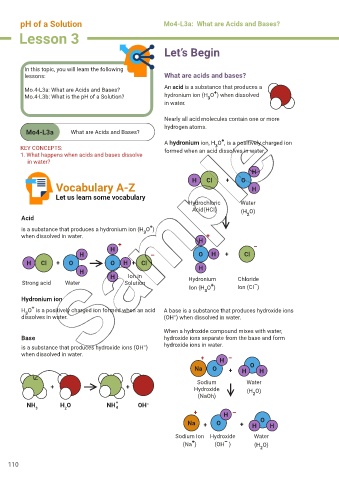
Mo.4-L3a: What are Acids and Bases? An acid is a substance that produces a
+
Mo.4-L3b: What is the pH of a Solution? hydronium ion (H O ) when dissolved
3
in water.
Nearly all acid molecules contain one or more
hydrogen atoms.
Mo4-L3a What are Acids and Bases?
+
A hydronium ion, H O , is a positively charged ion
3
KEY CONCEPTS: formed when an acid dissolves in water.
1. What happens when acids and bases dissolve
in water?
H
H Cl + O
Vocabulary A-Z H
Let us learn some vocabulary
Hydrochloric Water
Acid(HCI) (H O)
Acid 2
+
is a substance that produces a hydronium ion (H O )
3
when dissolved in water. H +
+ –
H
H – O H + Cl
H Cl + O O H + Cl
H H
H Ion in Hydronium Chloride
Strong acid Water Solution + –
Ion (H O ) Ion (CI )
3
Hydronium ion
+
H O is a positively charged ion formed when an acid A base is a substance that produces hydroxide ions
3
dissolves in water. (OH ) when dissolved in water.
–
When a hydroxide compound mixes with water,
Base hydroxide ions separate from the base and form
–
is a substance that produces hydroxide ions (OH ) hydroxide ions in water.
when dissolved in water.
+ H –
Na O + H O H
Sodium Water
+ + Hydroxide (H O)
(NaOh) 2
NH H O NH + OH –
3 2 4
+ H –
Na + O + H O H
Sodium Ion Hydroxide Water
–
+
(Na ) (OH ) (H O)
2
110