Page 119 - Science Course 2 (Book 1)
P. 119
Mo4-L3b: What is the pH of a Solution?
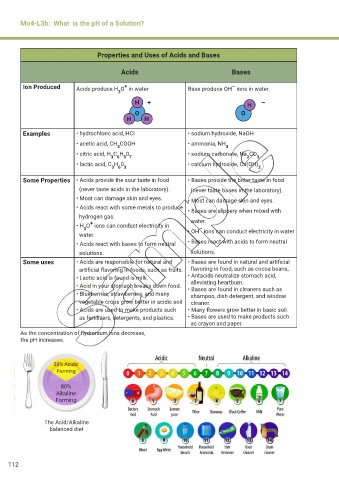
Properties and Uses of Acids and Bases
Acids Bases
+
Ion Produced Acids produce H O in water Base produce OH ions in water.
–
3
H + H –
O O
H H
Examples • hydrochloric acid, HCI • sodium hydroxide, NaOH
• acetic acid, CH COOH • ammonia, NH
3 3
• citric acid, H C H 0 • sodium carbonate, Na CO
3 6 5 7 2 3
• lactic acid, C H 0 • calcium hydroxide, Ca(OH)
3 6 3 2
Some Properties • Acids provide the sour taste in food • Bases provide the bitter taste in food
(never taste acids in the laboratory). (never taste bases in the laboratory).
• Most can damage skin and eyes. • Most can damage skin and eyes.
• Acids react with some metals to produce • Bases are slippery when mixed with
hydrogen gas. water.
+
• H O ions can conduct electricity in –
3
water. • OH ions can conduct electricity in water
• Acids react with bases to form neutral • Bases react with acids to form neutral
solutions. solutions.
Some uses • Acids are responsible for natural and • Bases are found in natural and artificial
artificial flavoring in foods, such as fruits. flavoring in food, such as cocoa beans,
• Lactic acid is found is milk. • Antacids neutralize stomach acid,
• Acid in your stomach breaks down food. alleviating heartburn.
• Bases are found in cleaners such as
• Blueberries, strawberries, and many shampoo, dish detergent, and window
vegetable crops grow better in acidic soil cleaner.
• Acids are used to make products such • Many flowers grow better in basic soil.
as fertilizers, detergents, and plastics. • Bases are used to make products such
as crayon and paper.
As the concentration of hydronium ions decrease,
the pH increases.
20% Acidic
Forming
80%
Alkaline
Forming
The Acid/Alkaline
balanced diet
112