Page 118 - Science Course 2 (Book 1)
P. 118
Mo4-L3a: What are Acids and Bases? Mo4-L3b: What is the pH of a Solution?
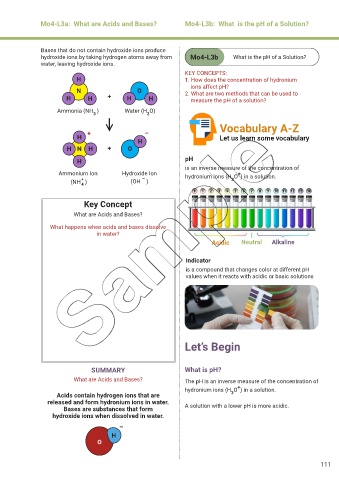
Bases that do not contain hydroxide ions produce
hydroxide ions by taking hydrogen atoms away from Mo4-L3b What is the pH of a Solution?
water, leaving hydroxide ions.
KEY CONCEPTS:
H 1. How does the concentration of hydronium
ions affect pH?
N O 2. What are two methods that can be used to
H H + H H measure the pH of a solution?
Ammonia (NH ) Water (H O)
2
3
Vocabulary A-Z
+ –
H Let us learn some vocabulary
H
H N H + O
H pH
is an inverse measure of the concentration of
Ammonium Ion Hydroxide Ion hydronium ions (H O ) in a solution.
+
–
+
(NH ) (OH ) 3
4
Key Concept
What are Acids and Bases?
What happens when acids and bases dissolve
in water?
Acidic Neutral Alkaline
Indicator
is a compound that changes color at different pH
values when it reacts with acidic or basic solutions
Let’s Begin
SUMMARY What is pH?
What are Acids and Bases? The pH is an inverse measure of the concentration of
+
hydronium ions (H O ) in a solution.
Acids contain hydrogen ions that are 3
released and form hydronium ions in water.
Bases are substances that form A solution with a lower pH is more acidic.
hydroxide ions when dissolved in water.
–
H
O
111